A bioengineer and neuroscientist at the University of California, Riverside, have received a $390,000 grant from the National Institutes of Health to develop a device to improve outcomes for patients suffering a severe stroke that requires a craniectomy to circumvent debilitative tissue swelling.
The device aims to relieve swelling in brain tissue and administer therapeutic amounts of neuregulin-1 (NRG-1), a molecule produced naturally by the body to regulate communication between cells in the brain and heart and promote their growth. The researchers expect the work will eventually lead to a new Food and Drug Administration-approved device for the treatment of ischemic stroke in humans.
Stroke occurs when blood flow to the brain is interrupted, reducing oxygen supply and killing brain cells. The most common and deadliest strokes are caused by blood clots that block the arteries leading to the brain. Known as ischemic stroke, these account for 80 percent of all strokes. The mortality rate for the most severe ischemic strokes runs as high as 80 percent, because the only treatment involves removing part of the skull, known as a craniectomy, to release pressure on the brain and wait for swelling to subside. But patient outcomes depend on how quickly medical interventions can reverse swelling, and every second lost risks a patient’s life.
Victor Rodgers, the Jacques S. Yeager, Sr. Professor of Bioengineering at UCR’s Marlan and Rosemary Bourns College of Engineering, developed a similar device with Dr. Devin K. Binder, a neurosurgeon and associate professor in residence at the UCR School of Medicine, to reduce swelling in brain tissue or spinal cord.
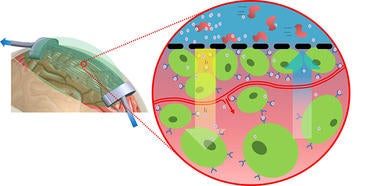
The device they invented consists of hollow fibers embedded in a flexible gel, which are attached to larger tubes on either end. After a portion of the skull is removed, the gel is placed on it, conforming to its shape. A solution is pumped into the tubes to encourage water molecules to move from the brain tissue and through the membranes of the tubes, a process known as osmosis, and out the opening at the other end. The osmotic therapy device reduces swelling quickly and has a patent pending.
Previous research by Byron Ford, a professor of biomedical sciences at UCR’s School of Medicine, showed that NRG-1 can protect brain cells from stroke damage in rats and mice. A single dose of NRG-1 was enough to prevent acute brain injury, most likely by blocking the ability of cells to become inflamed past the stroke site. The FDA has already approved clinical trials using NRG-1 to treat heart failure. The new research could hasten FDA approval of NRG-1 for treatment of stroke.
Rodgers and Ford will use the new grant to modify the existing osmotic therapy device to both draw out water and deliver NRG-1 into brain tissue. NRG-1 will flow through the device’s hollow tubes and diffuse through their membranes directly into the brain tissue without the need for needles or any other method of delivery. The researchers think that combining rapid swelling reduction with the neuroprotective effects of NRG-1 can improve outcomes for victims of the most severe ischemic strokes.
“We are hopeful that this technology will increase survival and improve the quality of life for patients suffering the devastating effects of severe stroke,” Rodgers said.
(banner photo credit: Idrewfedd on Wikimedia Commons)