What if you could cure cancer by re-engineering patients’ cells to better target and destroy their own tumors? With the advent of powerful new cellular engineering technologies, this is no longer the stuff of science fiction.
In the past few years, these technologies have enabled the development of revolutionary engineered cell therapies for treating cancer, such as CAR-T cell cancer immunotherapies for leukemia and lymphoma. They have also enabled development of treatments for rare genetic disorders, such as HSC gene therapies for “Bubble Boy disease” and beta thalassemia. Researchers worldwide are working at fever pitch to develop similar therapies for a large number of other deadly and debilitating illnesses.
But there’s a catch: with the cost of these so-called “living drugs” ranging from a few hundred thousand dollars to nearly $2 million, it’s unclear whether they’ll be sufficiently accessible to all those in need.
Now, in a watershed advance, engineers at the University of California, Riverside, in collaboration with researchers at City of Hope National Medical Center, have invented a device that holds potential for mass-producing engineered cells at lower cost, a tipping point for these lifesaving therapies.
In a new paper in the journal Nano Letters, a team of researchers led by Masaru Rao, an associate professor of mechanical engineering in the Marlan and Rosemary Bourns College of Engineering, describes a novel microfluidic device technology capable of addressing one of the most costly steps in the engineered cell therapy manufacturing process, namely gene delivery.
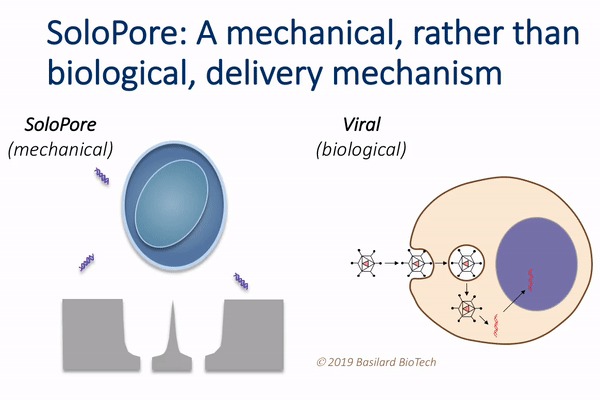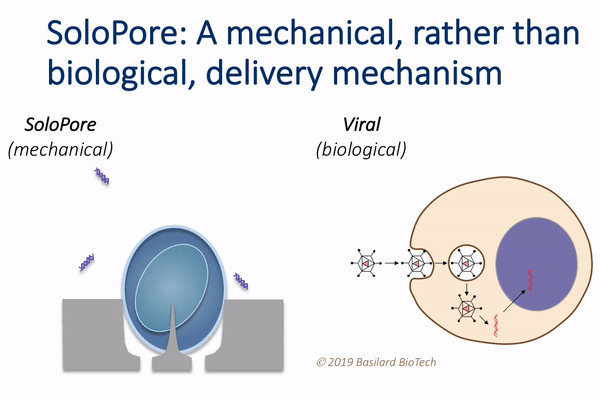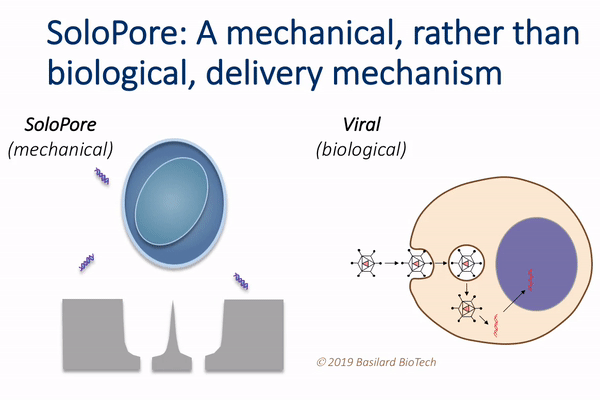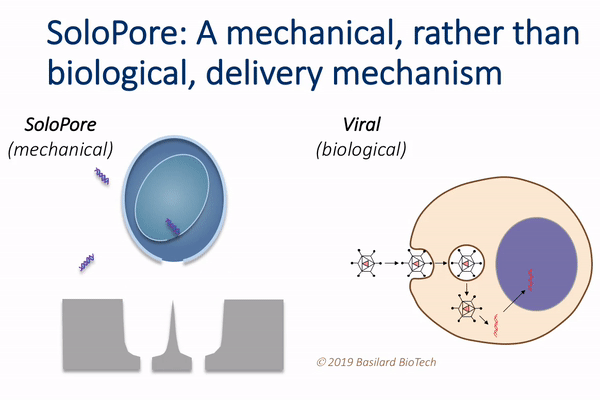
This technology, which the authors call deterministic mechanoporation, or DMP, uses fluid flow to pull each cell in a large population onto its own tiny needle. The flow is then reversed to release the cells from the needles, leaving a singular and precisely defined pore within each cell that allows for gene delivery.
“This simple, but elegant nanomechanical poration approach provides significant advantages relative to existing gene delivery techniques,” Rao said. “For example, since viral vectors make up a large fraction of the overall manufacturing cost of current cell therapies, their elimination through the use of DMP holds potential for considerable cost reduction.”
DMP’s unique single-site poration mechanism is key, since it minimizes damage to the cell, while producing a well-defined pathway for introducing genes. This provides opportunity for achieving both high delivery efficiency and cellular viability, which is difficult to achieve using other non-viral delivery techniques, such as electroporation.
“In fact, in our paper we show that DMP can engineer primary human T cells, the same kind of cells used in CAR-T therapies, with efficiencies that exceed a state-of-the-art electroporation tool by more than four-fold,” Rao said.
The DMP technology has been patented by UC Riverside and recently licensed to a new startup company that Rao has spun out of his lab, Basilard BioTech. The company is seeking to develop the technology, which it has branded SoloPore, as a disruptive new solution for engineering ex vivo cell and gene therapies for cancer specifically, as well as genetic disorders and degenerative diseases more broadly.
The paper, “Massively-Parallelized, Deterministic Mechanoporation for Intracellular Delivery,” is published in the journal, Nano Letters. In addition to Rao, authors include Harish Dixit, Renate Starr, Morgan L. Dundon, Pranee I. Pairs, Xin Yang, Yanyan Zhang, Daniel Nampe, Christopher B Ballas, Hideaki Tsutsui, Stephen J. Forman, and Christine Brown.