Two microscopic bubbles are better than one at penetrating soft materials, concludes a new study by engineers at the University of California, Riverside.
Optical cavitation, which uses a laser to form bubbles in a liquid that expand rapidly then collapse, could be a safe way to quickly and efficiently deliver therapeutic agents, such as drugs or genes, directly into living cells. Current methods for introducing foreign materials into cells, known as transfection, rely on puncturing the outer membrane with a laser, which risks heat damage to the cell, or a pipette, which risks contamination.
Though not quite ready for prime time yet, scientists are improving optical cavitation techniques. The new paper shows two bubbles produce long, fine jets that penetrate far enough with only five pulses to make cavitation potentially suitable for transfection or needle-free injections.
“The study of cavitation bubbles has evolved relatively fast, from learning how to avoid the damage they cause on ship propellers to benefitting medicine delivery,” said Vicente Robles, a doctoral student at the Marlan and Rosemary Bourns College of Engineering, who led the study. “The biggest limitation on their applications is our creativity.”
Cavitation bubbles are micron-sized and live for only a fraction of a second, but generate strong, local changes in physical properties of the surrounding medium, making them prime candidates for localized surface cleaning, cell targeting, and heating or cooling.
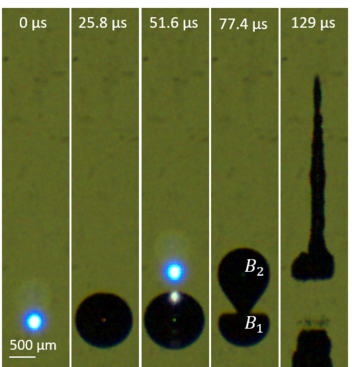
In double-bubble configurations, one bubble collapses faster and accelerates the neighboring bubble to invert and pierce itself, emitting a fast jet that could, if forceful enough, also pierce a cell membrane and possibly be used to transfect a cell. However, the jet’s speed, force, and trajectory are highly influenced by the mechanical properties of the medium surrounding it and the spatial and temporal separations of the bubbles.
Robles started by using lasers to create bubbles that form jets of water directed at a medium. He then compared single- and double-bubble jets directed at both petroleum jelly and a transparent agar gel widely used to model human tissue.
The double-bubble process created elongated, fast, focused jets that increased in length and volume when directed at the agar gel. Just five pulses penetrated 1.5 millimeters — enough to pierce human skin. This was achieved without the special micro-nozzles used in existing laser injection systems. In petroleum jelly, double-bubble jetting produced the same penetration length as single-bubble jetting, but with a 45% reduction in damage area, potentially resulting in less thermal and shockwave damage to the surrounding medium, and from three times farther away.
“The use of a laser-induced double-bubble arrangement is a significant advantage over previous studies, which rely on a converging nozzle or pressurized cavity to produce forceful jets,” mechanical engineering professor and senior author Guillermo Aguilar said. “Here, we take advantage of the inherent physics of the asynchronous collapse of two bubbles to accelerate the jet that pierces the nearby surface.”
The study concludes double-bubble cavitation could offer compact, device-free alternatives for needle-free applications after further study and improvement.
The paper, “Soft material perforation via double-bubble laser-induced cavitation microjets,” is published in Physics of Fluids. Other authors include E. Gutierrez-Herrera; L. F. Devia-Cruz; D. Banks; and S. Camacho-Lopez. The article was selected by the editors as an Editor’s Pick. The research was a collaborative effort between UC Riverside and the Center for Scientific Research and Higher Education at Ensenada, México, and supported by a Ford Foundation Predoctoral Fellowship to Robles.
Header photo by Juan Carlos González Parra