New insights into a little-understood DNA structure could provide the basis for new cancer therapies.
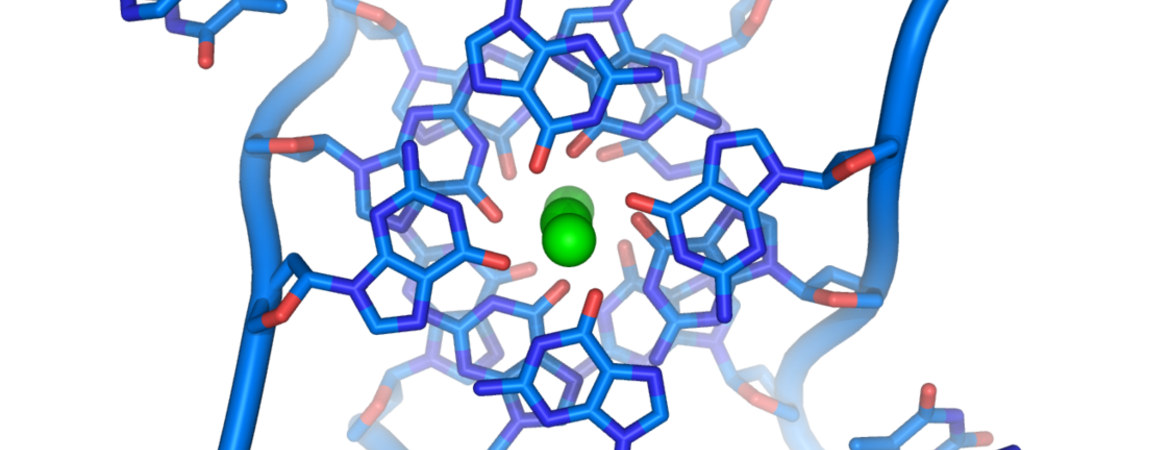
DNA is usually depicted as double-stranded, but not much is known about parts of the genome that adopt four-stranded structures known as quadruplexes. UC Riverside researchers have discovered that they play a key role in keeping cells healthy.
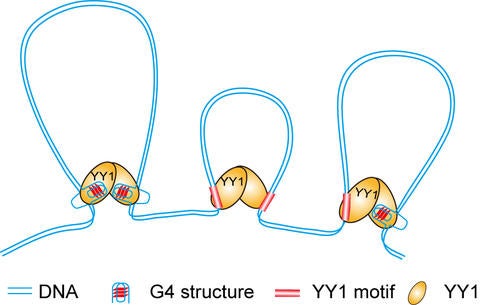
Specifically, the research identifies a protein that binds to these four-stranded structures, bringing together DNA segments that would otherwise remain far from one another, a process called DNA “looping.”
“DNA is like a rope, and from point A to point B is like a line. But if it knots, that’s looping,” said Yinsheng Wang, study lead and a distinguished chemistry professor at UCR. “One function of looping is to help regulate transcription, in which the DNA becomes messenger RNA molecules that go on to make proteins.”
If cells fail to make a sufficient amount of proteins, they could die. Alternatively, if some of them are produced in overabundance, the cell could live but may become cancerous.
The Wang Laboratory’s work in discovering how four-stranded DNA structures work with a particular protein to enable looping is detailed in the journal Nature Chemical Biology.
This protein is named Yin Yang1, or YY1, because it can turn some genes on and turn others off. YY1 is well-known as a type of protein that can control the rate of transcription. There are up to 1,600 proteins of this type in the human genome.
The Wang group, in collaboration with Jikui Song, a professor in the UCR Department of Biochemistry, was able to show that YY1 joins these quadruplex, four-stranded DNA regions together.
Researchers observed that YY1 can therefore regulate gene expression in two ways, both by interacting directly with quadruplex DNA in gene promoters – regions of DNA that controls gene expression – and by bringing remote quadruplex structures into close proximity of gene promoters through looping. These observations provide novel insights into the functions of YY1 and quadruplex structures in promoting healthy cell operations.
They also have important implications for biology research. YY1 is known to regulate genes related to cancer, and cancer cells also have increased levels of quadruplex DNA structures.
This work was supported by a R35 RIVER grant from the National Institute of Environmental Health Sciences.
The Wang Laboratory is focused on questions surrounding ways through which DNA gets damaged or mutates, and on the study of ways gene expression is regulated.
Going forward, Wang says he and his colleagues are interested in learning more about whether and how these elevated quadruplex DNA structures drive tumor progression.
“Potentially, this research could help cancer patients,” said Wang. “If you can find a way to disrupt YY1’s function in binding with quadruplex DNA, you might be able to modulate gene expression in tumor cells and slow their growth.”