Biological energy flows, such as in photosynthesis and respiration, depend on the transfer of electrons from one molecule to another. Despite its importance to sustaining life, factors governing the rate of electron transfer, especially over long distances, are not well understood because the systems that mediate such ultrafast processes are very complex. A better understanding of electron transfer rates would help scientists improve chemical transformations, energy conversion, electronic devices, and photonic technologies.
Now, an international team of researchers led by UC Riverside has observed picosecond charge transfer mediated by hydrogen bonds in peptides. A picosecond is one trillionth of a second. As short-chain analogs of proteins, crucially important building blocks of living organisms, peptides are chains of chemically linked amino acids. The discovery shows the role of hydrogen bonds in electron transfer. The results are published in Proceedings of the National Academy of Sciences.
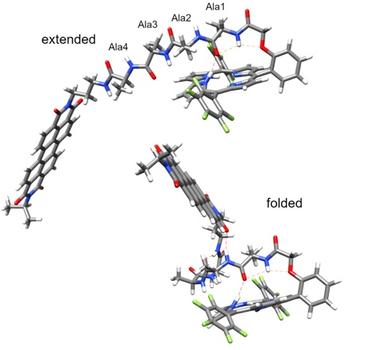
Valentine Vullev, a professor of bioengineering at UC Riverside’s Marlan and Rosemary Bourns College of Engineering, along with Daniel Gryko from the Polish Academy of Sciences, and Harry Gray from the California Institute of Technology, led a team that discovered unusually ultrafast electron transfer from a donor to an acceptor molecule connected with oligopeptide linkers stretching up to 20 covalent bonds. Electron transfer usually takes a microsecond, or one millionth of a second, in peptides with such long through-bond distances.
The researchers were surprised to observe picosecond electron transfer, a rate 1 million times faster than previously known for such systems.
“It shouldn’t work, but it does,” Vullev said. “The picosecond charge transfer we observed contradicts structural biology, assuming the expected random distribution of structures of the flexible peptide chains.”
The team chose donor and receptor molecules linked by short peptides they discovered actually assume well-defined structures stabilized by hydrogen bonds. Further analysis revealed that hydrogen bonds within each molecule brought the donor and acceptor close to each other in a scorpion-shaped molecular architecture, enabling picosecond electron transfer.
“This revolutionary design demonstrates short peptides can not only assume well-defined secondary conformations when templated by organic components but also provide a hydrogen-bonding network that can mediate electron transfer with unusually high efficiencies,” Vullev said. “Our work provides unprecedented paradigms for the design and development of charge-transfer pathways along flexible bridges, as well as insights into structural motifs for mediating electron transfer in proteins.”
The findings could lead to advances in energy storage as well as spur development of organic electronics that use conducting polymers instead of conducting minerals.
“One of the most exciting and fulfilling aspects about working in our group is being at the forefront of such discoveries and observing these spectacular results,” said co-author John Clark, a doctoral student in Vullev’s lab who did photochemical measurements for the research.
The paper, “Role of intramolecular hydrogen bonds in promoting electron flow through amino acid and oligopeptide conjugates,” is available here. Other authors include Rafał Orłowski, Olga Staszewska-Krajewska, Hanna Jedrzejewska, and Agnieszka Szumna at the Polish Academy of Sciences; John A. Clark, James B. Derr, Eli M. Espinoza, and Maximilian F. Mayther at UC Riverside; and Jay R. Winkler at the California Institute of Technology.