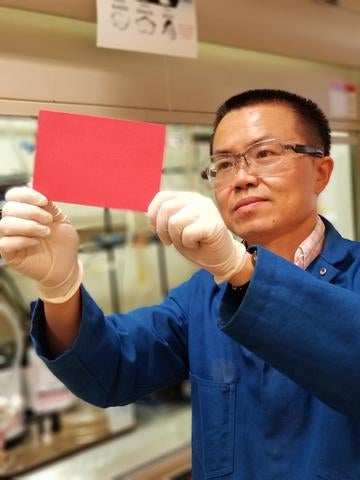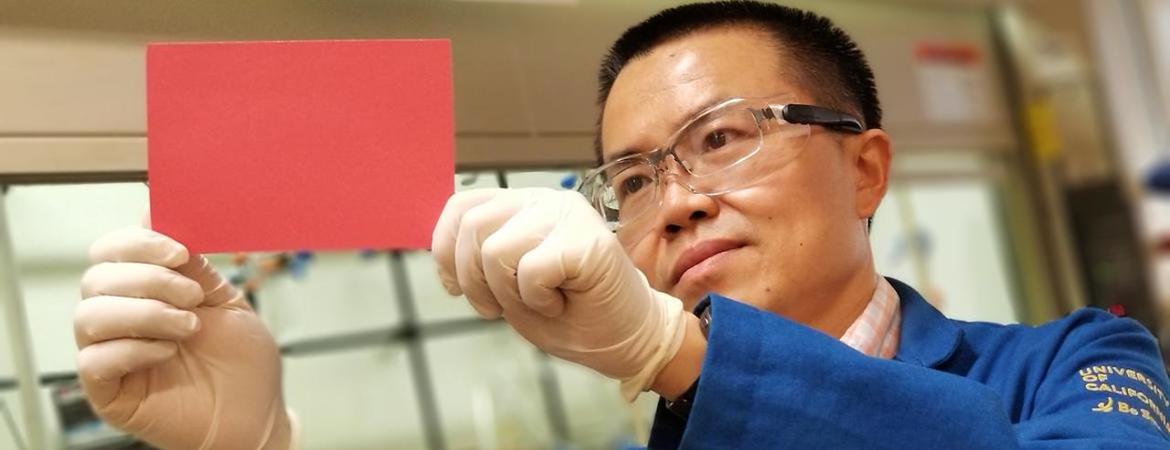
Imagine if water vapor in your breath or surrounding your fingertips revealed invisible patterns on commercial products — smartphones, laptops, expensive liquor — that verified the products’ authenticity and aided anticounterfeiting efforts.
Imagine, too, if fast, stable, and reversible color switching could be easily developed in solids, opening up promising applications in color displays, signage, sensors, and information encryption.
A team led by a chemist at the University of California, Riverside, has brought this fantasy closer to reality by fabricating for the first time “plasmonic” color-switchable films of silver nanoparticles, or AgNPs. Until now, such color changing of nanoparticles was mainly achieved in liquids, limiting their potential for practical applications.
“Rapid and reversible tuning of plasmonic color in solid films, a challenge until now, holds great promise for a number of applications,” said Yadong Yin, a professor of chemistry, who led the research team. “Our new work brings plasmonic metal nanoparticles to the forefront of color-converting applications.”
Study results appear in Angewandte Chemie International Edition. The research paper has been designated a VIP paper by the journal.
Plasmonics
Plasmonic metal nanoparticles, such as gold and silver, have special optical properties because they efficiently absorb and scatter light at particular wavelengths. Their colors can be altered by changing the distance between their individual particles — a feature that Yin’s research team took advantage of to develop their plasmonic color-switching film.
The researchers coated a glass substrate with a layer of sodium borate, or borax. Then they sprayed AgNPs over the borax to form a film. Yin explained that each AgNP has capping ligands on its surface that introduce distance between the AgNPs. Without the buffer provided by the ligands, the nanoparticles would clump together.
Chemistry lesson
In the presence of water or moisture, borax turns to boric acid and releases hydroxyl ions. These ions “deprotonate” a chemical group of the ligands, resulting in the loss of a proton and the addition of a negative charge on the AgNPs. Repulsion forces push the negatively charged nanoparticles away from each other. The nanoparticles, which are pink, acquire new interparticle distances, causing them to reflect a different color: yellow.
When the moisture is removed, the boric acid converts back to borax by capturing hydroxyl ions, initiating a protonation of the ligand’s chemical group. This causes a reduction in surface charges on the ligand, weakening the repulsion forces between the AgNPs and causing them to draw closer to each other and aggregate. With interparticle distances now reduced, the color of the AgNP film switches back from yellow to pink, demonstrating full reversibility.
“Through this mechanism, we could rapidly achieve plasmonic color switching of the AgNP film in the presence or absence of moisture,” Yin said. “In our experiments, we exposed the AgNP film to moisture of 80% relative humidity and found the film changed colors from pink to red, orange, and finally yellow.”
By the fingertips
Making use of the relative humidity around human fingers — as high as 100% — Yin’s team found AgNP films can change color in response to the proximity of a fingertip.
“This allows for a convenient, rapid, and touchless method that can be used in information encryption and product authentication,” Yin said. “Various high-resolution patterns can be effectively encrypted in the AgNP films through a lithography process and then decrypted when exposed to moisture in human breath or from fingertips. Other foreseeable applications include secure communication and calorimetric real-time environment or health monitoring.”
Yin’s team found that the moisture-responsive AgNP films showed reversibility and repeatability in plasmonic color switching for more than 1,000 cycles.
The research was supported by a grant to Yin from the U.S. National Science Foundation. He was joined in the research by Rashed Aleisa and Ji Feng of UC Riverside; and Luntao Liu, Yun Zhang, Yiqun Zheng, and Wenshou Wang of Shandong University, China.