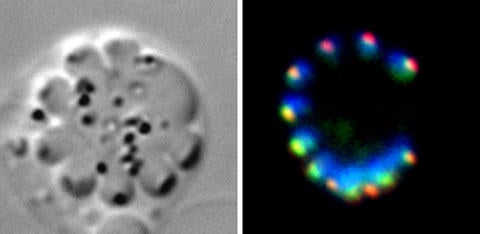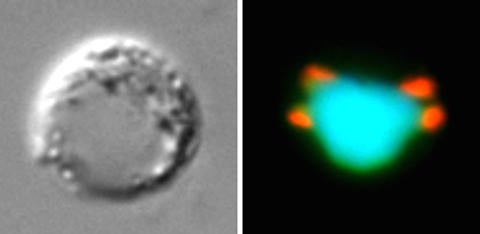
Scientists have made a major breakthrough in understanding how the parasite that causes malaria is able to multiply at such an alarming rate, which could be a vital clue in discovering how it has evolved and how it can be stopped.
For the first time, scientists have shown how certain molecules play an essential role in the rapid reproduction of parasite cells and cause this deadly disease. If experts can prevent the malaria parasite from reproducing, it could help eradicate one of the world’s biggest killer infections.
The research, co-led by Karine Le Roch of the University of California, Riverside, and Rita Tewari of the University of Nottingham, appears in Cell Reports.
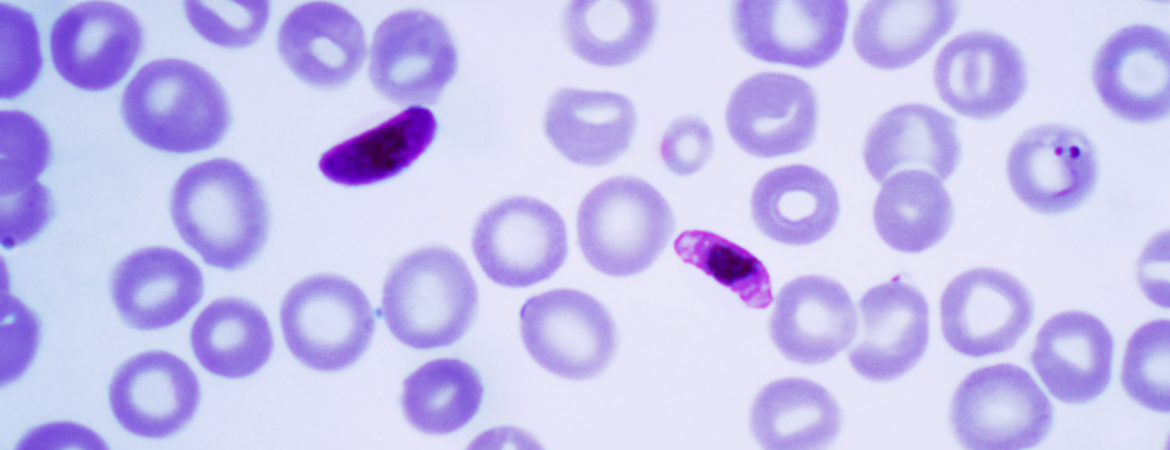
Malaria is responsible for almost half a million deaths a year, mainly in tropical developing countries. The disease is caused by a one-celled parasite called Plasmodium that is transmitted from person to person through female Anopheles mosquitoes. The insects, which bite humans to get blood needed to nurture their eggs, ingest the parasite after biting infected individuals. The parasites then reproduce, multiply, and develop inside the mosquito.
As part of its latest research, the team wanted to better understand how the parasite’s cell divides and multiplies, especially within a mosquito.
“By understanding the fundamental aspect of parasite biology, we are decrypting how the parasite divides, and how the different mechanisms regulating cell division can affect the ability of the parasite to thrive and replicate exponentially inside its hosts,” said Le Roch, a professor of molecular, cell and systems biology and director of the UC Riverside Center for Infectious Disease and Vector Research. “If we identify the molecular components that are essential for the replication of this parasite, we will be able to develop novel and long-lasting therapeutic strategies against this devastating disease.”
Proteins are large, complex molecules that play many critical roles in the body. They do most of the work in cells and are required for the structure, function, and regulation of the body’s tissues and organs. Each organism has DNA organized into a certain number of chromosomes and needs condensins in order to ‘split’ this DNA when cells multiply. Condensins are large protein complexes that play a central role in chromosome assembly and segregation during mitosis and meiosis.
In Plasmodium, the role of condensins in multiplication and proliferation was unclear. The team looked at two crucial condensin subunits, SMC2 and SMC4, which are required to maintain the structure of chromosomes in a cell of other organisms.
“We have tried to understand how these molecules work in the unusual pattern of multiplication by the parasite,” said Tewari, a professor of parasite cell biology at the University of Nottingham. “We found these molecules are present at all the stages of multiplication and only at a certain part of the chromosome: the centromere. We wanted to understand how the parasite multiplies, how these molecules organize themselves and the DNA in those cells. It is fascinating how a single cell can carry out so many different modes of multiplication, and we need to understand how it does this.”
After analyzing the parasite, the team found the malaria parasite has evolved to ensure its survival by way of an unusual type of cell division.
“Over time, this particular parasite has adapted to survive and has a lot of genetic plasticity, which is why it is difficult to control the disease,” Tewari said. “We need to understand what gives the parasite this plasticity and what it needs at every stage to survive, so it is crucial to understand how the parasite cell divides. The aim of our research is not to develop a drug immediately, but to answer the fundamental question of how the parasite divides and survives and the machinery it uses. The parasite has diverse modes of multiplying, so even if a drug or an effective vaccine is created, the parasite may be able to adapt, and we need to understand how. This is a next step toward that goal.”
Le Roch and Tewari were joined in the research by scientists at the University of Dundee, University of Warwick, and The Francis Crick Institute, United Kingdom; University of Bern, Switzerland; and the International Center for Genetic Engineering and Biotechnology, India.
Le Roch was supported by grants from the National Institute of Allergy and Infectious Diseases and UC Riverside.
Above is a modified version of the University of Nottingham news release written by Charlotte Anscombe.