A discovery by UC Riverside scientists could assist water providers across the nation as they face new federal standards to limit “forever chemical” concentrations in drinking water.
Known by scientists as PFAS, or poly- and perfluoroalkyl substances, forever chemicals have been used in thousands of products, ranging from potato chip bags to fire suppressant foams. However, they are now being phased out because they have leached into groundwater supplies and are linked to certain cancers and other health maladies.
Earlier this year, the U.S. Environmental Protection Agency imposed water quality limits that restrict certain forever chemicals to only 4 parts per trillion in the nation’s tap water, spurring water providers to find PFAS cleanup solutions.
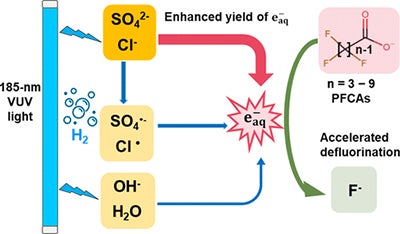
A UCR team led by Haizhou Liu, a professor of chemical and environmental engineering, discovered a chemical process that allows high levels of salt normally found in wastewater from water treatment plants to act as a catalyst that facilitates the breakup of PFAS compounds by cleaving the stubbornly strong fluorine-to-carbon bonds. Normally, salt in wastewater impedes the cleanup of chemical pollutants. This solution to PFAS pollution is detailed in the journal Environmental Science & Technology.
The work builds on Liu’s discovery in 2022 that PFAS compounds can be destroyed in a one-step treatment by irradiating water with short-wavelength ultraviolet light via tuning a process that does not require additional chemicals or leave behind toxic residuals. Both works are protected by patents.
“We were looking at PFAS with different carbon chains, short chains, and we also looked at salty wastewater that has a high concentration of chloride and sulfate,” Liu said. “The results show that the salinity in wastewater acts as a catalyst when receiving the UV light to make this process even more effective and much faster.”
Liu said the process extremely efficient at PFAS destruction because the short-wavelength ultraviolet light (which is distinct from traditional UV light used for water disinfection) is not quenched by undesirable chemicals in the wastewater.
“It not only destructs long-chain PFAS, but also short chains PFAS that are more difficult to get rid of by traditional separation technologies,” Liu said.
The breakthrough by Liu’s team is expected to benefit municipal and privately owned water providers that use or plan to use what is known as “ion exchange” technology to separate PFAS compounds from drinking water supplies that create brine waste containing PFAS pollutants.
In ion exchange water treatments, negatively charged PFAS molecules are exchanged with negatively charged
ions on the surface of tiny resin beads in large treatment tanks. This causes the PFAS pollutants to glom onto the beads. Over several months, however, resin beads become ineffective because they get saturated with PFAS molecules. The resin beads can be regenerated and reused by flushing them with saltwater. This creates a brine wastewater with heavily concentrated PFAS pollution that now can be treated with Liu’s process.
The utility of Liu’s process goes beyond permanently destroying PFAS from ion exchange treatment plants. Brine wastewater containing toxic PFAS is also produced by water treatment technologies that use membrane reverse osmosis filtration technologies. Additionally, landfill leachate wastewater, salty industrial wastewater produced from chemical manufacturing processes that use fluorine polymers, and brackish groundwater impacted by PFAS pollution can be cleansed using the method discovered by Liu and his team.
The title of Liu’s study is “Promotive Effects of Chloride and Sulfate on the Near-complete Destruction of Perfluorocarboxylates in Brine via Hydrogen-tuned 185-nm UV Photolysis: Mechanisms and Kinetics.” In addition to Liu, its authors are Sitao Liu, Gongde Chen, Qingyang Shi, Jay Gan, Bosen Jin, and Yujie Men, all affiliated with UC Riverside.
This research was supported by the U.S. National Science Foundation PFAS-ERASE Initiatives (CBET-2131745) and I-Corps Program (TIP-2310201), and UC Riverside Proof-of-Concept 400 Grant.
Header photo: Ion exchange tanks use for drinking water treatment. (Getty Images)