Scientists at the University of California, Riverside, have discovered how adult stem cells retain their regenerative power. The researchers demonstrate in a paper published in the journal Genes & Development that these cells rely on a group of helper proteins called histone chaperones — organizers or guides known to help package our two-meter-long genome when cells need to divide or switch on specific genes.
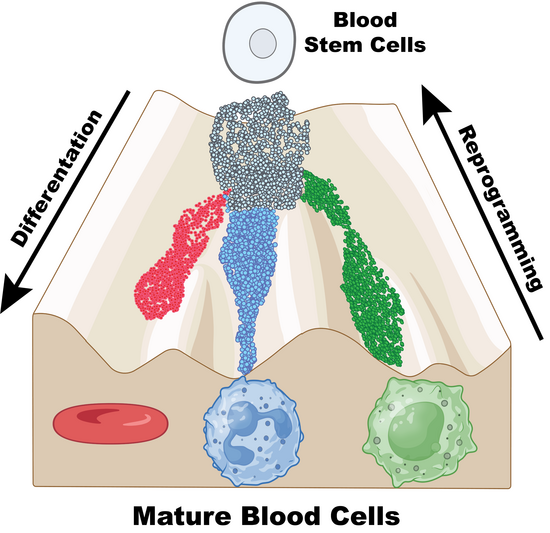
Every organ in our body contains a small number of adult stem cells that are specific to its niche. These cells act like a backup system. When needed, they divide to make copies of themselves or generate mature cells such as neurons or red blood cells that replenish our organs and tissues.
The study, which used mouse stem cells, found that when certain histone chaperones stop working, stem cells lose their ability to renew themselves and instead start turning into specific cell types. This loss in renewal capacity is caused by changes in the way specific genomic regions are packaged. According to the researchers, this could help develop stem cell-based therapies to heal damaged tissues, treat diseases, or even fight aging.
The research will be featured on the cover of the May 2025 issue of Genes & Development.
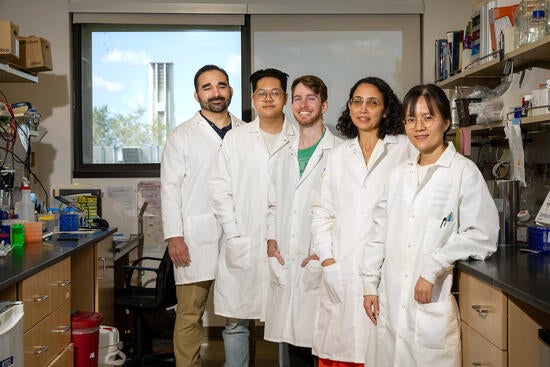
Sihem Cheloufi, an assistant professor of biochemistry at UC Riverside, led the research team. She has been fascinated by cellular reprogramming since high school after learning about Dolly the sheep, the first cloned mammal to be created from an adult cell.
“That discovery sparked my interest in epigenetic reprogramming, the idea that we can change a cell’s behavior without changing its DNA,” she said. “Now, with breakthroughs in CRISPR, AI, sequencing, and other technologies, we’re able to explore these questions more deeply than before. Stem cells are at the heart of development, aging, cancer biology, and regeneration. Our study shows how histone chaperones maintain stem cell identity, making them pivotal for advancing stem cell-based therapies.”
The researchers focused on blood-forming stem cells, which copy themselves and create immune cells that help defend the body from infection. The team explored about 25 different histone chaperones and found that all of them contribute to stem cell maintenance but in different ways. Two candidates stood out: CAF-1, a chaperone that works during genome duplication, and SPT6, a chaperone that works when genes are turned on.
The team found that when they disrupted CAF-1 and SPT6, the adult stem cells stopped dividing normally and began maturing instead into specific types of blood cells, suggesting that scientists may be able to guide stem cells into becoming desired cell types simply by manipulating histone chaperones.
Reuben Franklin, a postdoctoral fellow participating in the UCR California Institute of Regenerative Medicine scholar training program, continued working on histone chaperones and cell fate in Cheloufi’s lab after earning his doctoral degree at UCR. He explained that the most striking finding of this study is how manipulating histone chaperones, which play multiple roles in cellular processes, can lead to very specific changes in stem cell identity.
“Given that histone chaperones are common to all cells, it will be exciting to extend our work to other stem cell systems,” he said. “In this study, we found depleting CAF-1 led to a ‘mixed cell state’ where cells display traits of multiple distinct cell types, while loss of SPT6 triggered specific differentiation pathways. As gene and cell therapies evolve, these two histone chaperones could be targeted to influence cell identity and potentially guide stem cells into specific lineages. This is key for developing future therapies.”
Brian Zhang, a student in UCR’s Cell, Molecular, and Developmental Biology Graduate Program, is the research paper’s co-first author along with Franklin. He used single cell sequencing technology to reveal what the stem cells were turning into after the activity of histone chaperones was disrupted.
“Biology poses some of the most complex questions,” he said. “Understanding why cells behave the way they do — what governs their actions — is what drew me to this field. It’s exciting to be part of a team that works to better understand how to control stem cell behavior, which could be leveraged for therapies focused on tissue regeneration or disease treatment.”
The study was supported by grants from the National Institute of General Medical Sciences and the University of California Office of the President Cancer Research Coordinating Committee, as well as traineeship support from the California Institute of Regenerative Medicine and UCR’s RISE & McNair undergraduate research programs.
Cheloufi, Franklin, and Zhang were joined in the study by other members of the Cheloufi lab, including a senior undergraduate student, Jonah Frazier, and collaborators from the UCR Department of Biochemistry; Massachusetts Institute of Technology; Massachusetts General Hospital; Cold Spring Harbor Laboratory in New York; and Yonsei University in Korea.
The paper is titled “Histone Chaperones Coupled to DNA Replication and Transcription Control Divergent Chromatin Elements to Maintain Cell Fate.”
Header image credit: anusorn nakdee/iStock/Getty Images Plus.